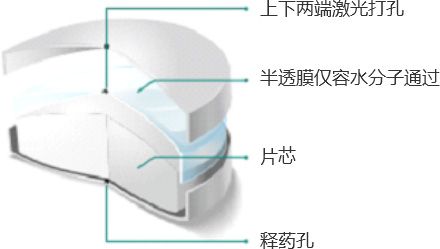
(1)Permeation pump technology
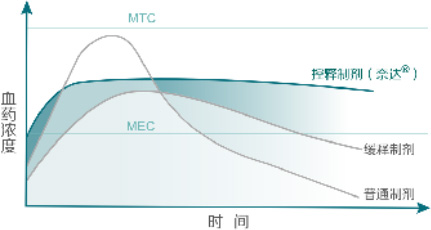
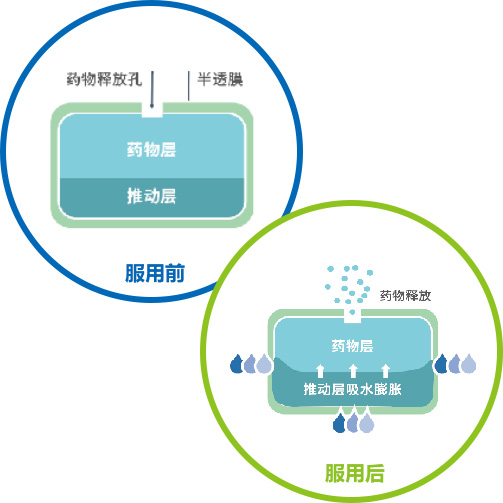
The osmotic pump coating technology relies on osmotic pressure regulators to produce a constant osmotic pressure effect, releasing drugs at a constant rate of 24 hours. This is the advantage that distinguishes it from ordinary tablets or skeleton sustained-release tablets. The advantages of osmotic pump drugs in clinical practice are that the blood drug concentration in the body is more stable, the therapeutic effect is more long-lasting, the blood drug peak phenomenon is avoided, and safety is improved. In addition, patients have a higher compliance with medication, taking it once a day, reducing the risk of missed or multiple doses.
Qingdao Baiyang Pharmaceutical has mature osmotic pump coating technology, which is divided into single chamber and double chamber osmotic pumps. Our company has independently developed multiple products and successfully launched them for production and commercial sales. For example, the single chamber osmotic pump technology products include metformin hydrochloride sustained-release tablets (III) and carbamazepine sustained-release tablets (II) (commissioned production), and the double chamber osmotic pump technology products include nifedipine controlled-release tablets and glipizide controlled-release tablets (commissioned production). The product development adopts the concept of "quality comes from design", and conducts systematic and scientific evaluation and research on key material properties such as CMA (permeability pump functional polymer, etc.) and key process parameters CPP, using DOE design to ensure stable quality, controllable quality, reproducible process, and continuous quality improvement throughout the product lifecycle.
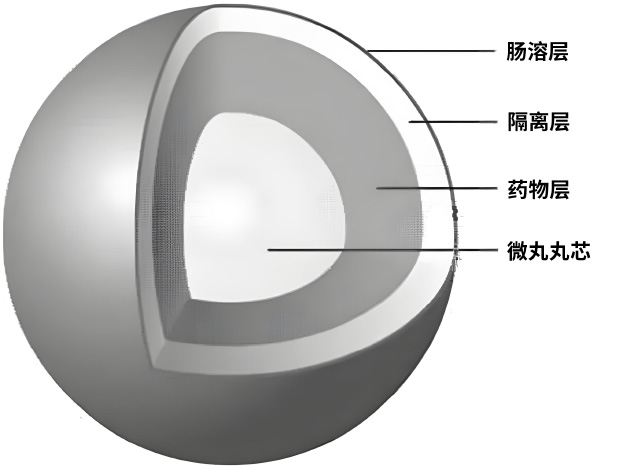
(2)Micro pill sustained-release coating technology
The sustained-release coating technology of microspheres can achieve controlled drug release, intestinal localization or targeted administration, pulse administration, timed or programmed administration, mask taste and improve taste, and significantly reduce the toxic side effects of drugs. This technology can be made into capsules, tablets, and dry suspensions. For example, the sustained-release capsules of Meverine Hydrochloride can be controlled and administered once a day, and can be effective for 24 hours; Duloxetine hydrochloride enteric coated capsules can be delivered through intestinal targeted administration, avoiding gastric irritation and degradation by gastric acid, improving drug stability, and ensuring drug safety.